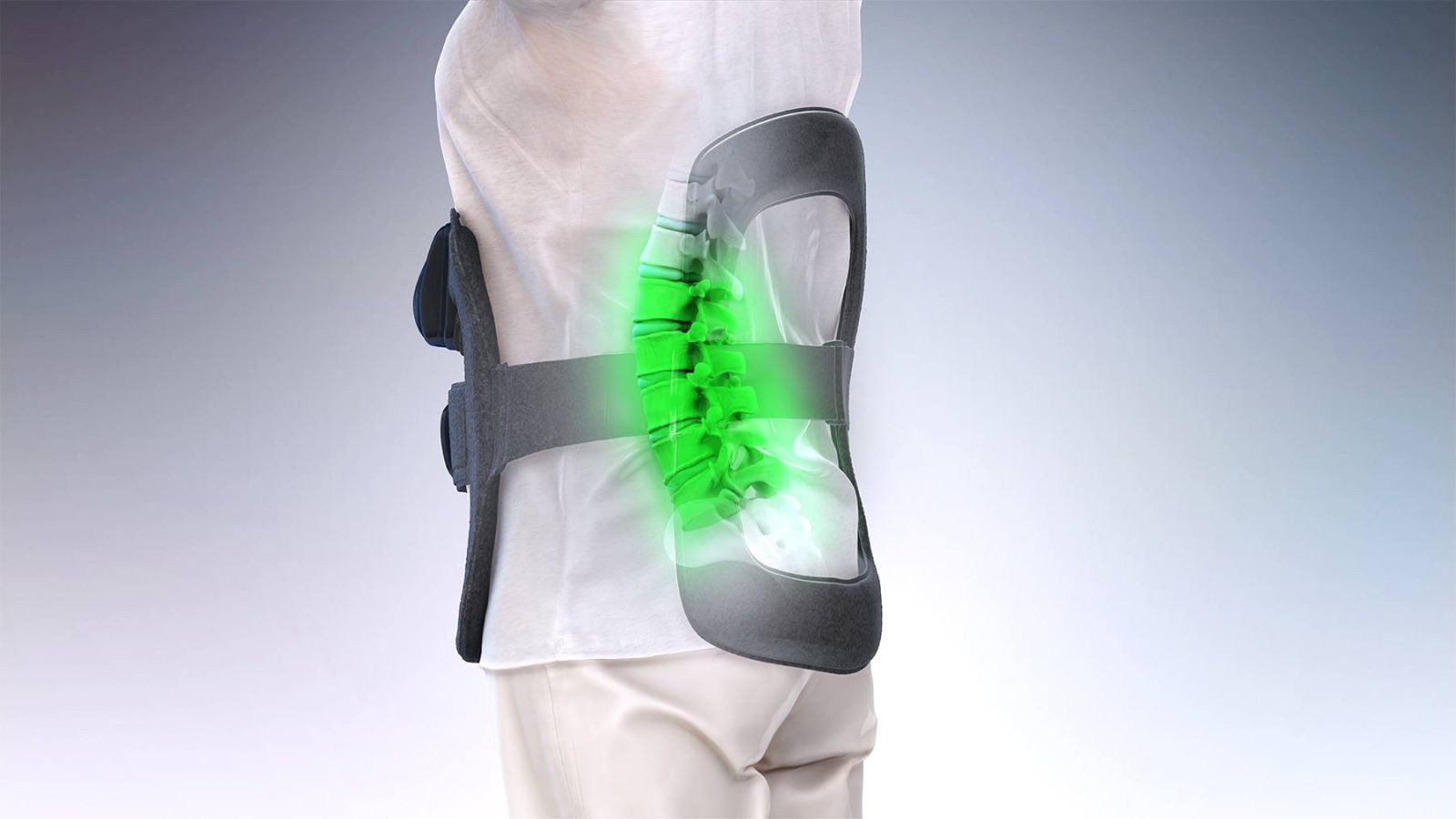
Spinal fractures pose a dual challenge: restoring stability, while supporting bone healing strong enough for long-term recovery. While traditional bone grafts have long been central to fusion in fracture repair, advances in biologics are transforming the field. Dr. Larry Davidson, a board-certified neurosurgeon with fellowship training in complex spinal surgery, notes that biologics, materials designed to stimulate or support bone growth, provide surgeons with new tools to reduce complications and improve outcomes.
These advances do more than replace missing bone. They create biological environments that promote healing, reduce the risk of non-union and improve the likelihood that patients recover with durable corrections. From bone grafts to growth factors and engineered scaffolds, biologics are now integral to modern fracture care.
The Role of Bone Grafts
Bone grafts have traditionally been used to provide a scaffold for new bone growth, helping fractured vertebrae fuse into stable structures. Autografts, harvested from the patient’s own pelvis or ribs, remain the gold standard due to their natural compatibility and growth potential. Yet harvesting autografts carries risks, such as donor site pain and added surgical time.
Allografts, taken from donor bone, provide alternatives, without the complications of harvesting. While they lack the same biological activity as autografts, they still offer structural support and have been refined through sterilization and processing methods, that reduce rejection. Synthetic grafts, made from ceramics or polymers, further expand options by mimicking bone’s physical properties. These choices give surgeons flexibility, but biologics extend beyond scaffolding to active stimulation of healing.
Growth Factors and Their Impact
Growth factors represent a major advancement in spinal fracture repair. These proteins regulate cell activity, encouraging bone-forming cells to migrate, proliferate and create new bone. Bone Morphogenetic Proteins (BMPs) are the most studied and widely used.
BMPs can be delivered to the fracture site, where they stimulate rapid and robust bone formation. They are especially useful in cases where natural healing potential is compromised, such as in older patients or those with osteoporosis. Other factors, including Platelet-Derived Growth Factor (PDGF) and Vascular Endothelial Growth Factor (VEGF), also play roles in enhancing bone and blood vessel growth. Growth factors are most powerful when combined with graft materials, creating an environment that not only supports, but also accelerates fusion. Their ability to reduce non-union rates makes them an important tool in complex fracture repairs.
Engineered Scaffolds and Biocompatible Materials
Engineered scaffolds combine structural support with biological activity. These materials are designed to integrate seamlessly with patient anatomy, offering strength, while encouraging bone growth. Some scaffolds are coated with growth factors, or seeded with stem cells, to further enhance healing.
Biocompatible materials, such as calcium phosphate and bioactive glass, mimic bone’s mineral structure, providing natural templates for new growth. These materials are resorbable, meaning the patient’s own bone gradually replaces them during healing. These innovations broaden treatment options for patients with severe fractures or low bone quality, supporting healing throughout the recovery process.
Stem Cells in Fracture Repair
Stem cells represent another frontier in biology. Mesenchymal Stem Cells (MSCs), harvested from bone marrow or fat tissue, have the potential to differentiate into bone-forming cells. When applied to fracture sites, they contribute directly to healing, while releasing signals that stimulate surrounding tissues.
Although still largely experimental, stem cell therapies are showing promise in clinical studies. For patients with complex fractures or impaired healing capacity, these approaches may provide solutions, where traditional methods fall short.
Reducing Complications with Biologics
Non-union, the failure of bones to heal, is a major complication in spinal fracture repair. Biologics address this risk by strengthening the fracture’s biological environment. By combining scaffolds, growth factors and stem cells, surgeons can reduce the likelihood of fusion failure and improve patient outcomes. Biologics do not replace surgical technique, but enhance it. When used thoughtfully, they complement precise instrumentation and careful planning, creating conditions for more reliable healing.
Dr. Larry Davidson remarks, “There’s still work to be done, but we’re on the right path.” His observation reflects how biologics, while not yet perfect, represent a major step forward in reducing complications, and improving long-term success in fracture repair.
Athletes and Biologic Strategies
Athletes recovering from spinal fractures face unique pressures to return to activity quickly. Biologics may shorten healing times, by accelerating fusion and reducing pain. Growth factors and biologically active scaffolds, when paired with rehabilitation, can help athletes resume training with greater confidence. For this group, biologics represent not just stronger healing, but faster restoration of performance potential. Surgical strategies incorporating these tools balance the need for stability with the goal of returning to competition.
Rehabilitation and Biologic Support
Rehabilitation after fracture repair remains critical, but biologics can make the process more effective. By improving fusion rates, they allow patients to begin therapy sooner, and progress with less risk of setbacks. Stronger healing translates into faster gains in mobility and independence.
Physical therapy, combined with biologically enhanced fusion, supports outcomes that extend beyond pain relief to include endurance, flexibility and strength. Patients benefit from recovery plans that integrate surgical and biologic innovations, with structured rehabilitation.
Patients as Partners in Biologic Care
Patients benefit from understanding the role biologics play in their treatment. Educating patients about graft choices, growth factors and stem cell options allows them to participate in shared decision-making. This transparency builds trust and helps align care with patient values and expectations. When patients feel informed, they are more likely to adhere to recovery plans and appreciate the broader goals of biologically supported healing.
Research in biology continues to expand. Next-generation materials may combine growth factors, stem cells and engineered scaffolds into integrated systems, tailored to individual patients. Advances in 3D printing could allow for custom biologic implants that match patient anatomy precisely. It means safer surgeries, faster healing and stronger outcomes for patients. For surgeons, it provides an expanding toolkit to manage fractures that once carried high risks of complications.
Biologics are transforming spinal fracture repair, providing options that go beyond traditional grafts, to actively support healing. Growth factors, engineered scaffolds and stem cells help strengthen fusion, reduce complications and speed recovery. This progress underscores the importance of thoughtfully integrating biologics into surgical care. It reflects a broader evolution in spine surgery, where advances in biological science work hand in hand with surgical expertise, to give patients the best chance at a strong, lasting recovery.